Medical Document Management Software: All You Need to Know

Key takeaways
- Is healthcare DMS the solution that can handle it all? Figure out how a modern DMS can enable seamless document management with tools for document capture, storing, distribution, tracking, and many others.
- The rapid growth of the organization, a decrease in personnel productivity, compliance, and regulatory challenges are just a few signs that the medical organization highly requires the integration of medical documentation software. Check out all the scenarios when having such software is a must.
- Improved security, faster document retrieval, elimination of human error, reduction of costs, and many other benefits can be unlocked with medical DMS. Discover the full range of advantages that you can get with such a solution.
- Top medical DMS on the market: features, OCR, the scale of workflow management, and detailed overview of other key characteristics.
- Explore our expertise in DMS development using Alfresco, one of the most robust platforms for building such software.
How do we act when we have a headache? Right, we take a painkiller and wait for a long-awaited relief. But what if we think more globally, in terms of an enterprise level, when suddenly the necessary documentation is missing, papers are piling up drastically, and the whole workflow is a complete mess? Is there any solution that cures the situation as effectively as a painkiller?
Medical organizations with progressive vision opt for a software savior called document management system, the technology that simplifies the main troublesome processes caused by paper documentation. Thus, continue reading this article, and you will figure out why medical institutions require a properly set up medical documentation software, how the whole solution functions, and in which way to develop one that will meet your requirements most efficiently.
What is document management software?
Technologies that can streamline the entire workflow of the company are rapidly grabbing the attention of numerous companies, especially in the medical sector, where the work with documents should be fast, secure, and optimized. A bright example of such a highly-efficient solution is a document management system. But what exactly is it, and what are its core features? Before answering this question, we offer to explore the main types of medical documents that such software help handle.
The main types of medical documents that DMS helps manage
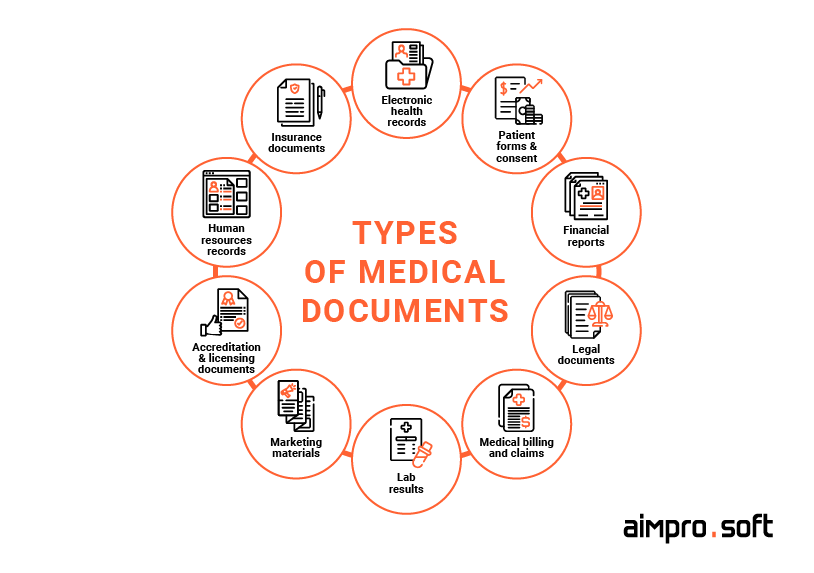
Types of medical documents
Medical documentation encompasses data about a wide range of the organization’s activities, including info about treatments, test results, medical history records, financial records, and many others. It’s usually categorized into clinical (documents about patients and their treatment) and non-clinical (documents of administrative nature). Overall, medical documentation can be divided into the following general sub-categories:
Electronic health records
Electronic health records are digital versions of a patient’s medical record that include details about their current health and medical history. To enhance workflow efficiency, DMS can be integrated with an existing EHR system and retrieve patient health information from it. DMS can also help guarantee data security by giving access controls and audit trails that track who and when accesses patient records.
Medical reports
Medical reports, including notes and summaries, is another commonly met type of medical document. With DMS, healthcare providers can use optician character recognition to scan physical versions of documents and transform them into digital ones or voice recognition software to dictate notes, which are then transcribed and stored in the DMS. This can save time and reduce the risk of errors associated with manual transcription. DMS can also enable enhanced confidentiality of the transcribed documents.
Patient forms
DMS can assist with the management and storage of patient consent forms, HIPAA forms, and other connected documents. Healthcare providers can use DMS to electronically record patient signatures on consent forms, removing the need for paper forms.
Insurance and claims
DMS can be used for managing medical billing and claims papers, such as insurance claims, invoices, and receipts. DMS allows healthcare providers to electronically submit claims to insurance providers, saving time and effort that would otherwise be needed for manual submission. DMS can also monitor the status of claims and payments, giving you a better understanding of the revenue cycle.
Compliance and regulations
DMS can help with the management of compliance and regulatory papers like medical licenses, certifications, and accreditation documents. Healthcare organizations can use DMS to guarantee that all necessary documents are up-to-date and easily accessible. DMS can also send alerts and notifications when papers need to be renewed or updated, ensuring that the organization stays in compliance with legal mandates.
Financial documents
Invoices, budget reports, financial statements, and bills are common forms of financial, medical paperwork. DMS can be leveraged to streamline financial document management, reduce the risk of errors and fraud, and improve overall financial efficiency and accuracy. It can also be integrated with the medical facility’s accounting system for a more efficient document flow.
Although the types of medical documents are not limited to the ones mentioned above, we aimed to cover the most commonly met categories. And as you can see, there is a vast range of medical documents that circulate within various types of healthcare organizations. Be it a private practice, health clinic, or a hospital, DMS features can simplify the work with document flow. To explore what functionality makes such systems so efficient, check out the section below.
Healthcare DMS features: a detailed overview
Having explored the main types of medical documents, let’s find out finally what healthcare DMS is. The document management system is a rather multi-functional tool that can be perceived from various perspectives:
System for document digitizing & capturing
DMS speeds up the working processes of the company by easily converting documents into their digital analogs via scanning (bulk, Optical Character Recognition, microfiche and microfilm scanning, etc.). When it’s completed, the documents can be converted into PDF, JPG, and other standard formats that make documentation tracking seamless.
Tool for document storing
DMS can free up space that you use for storing voluminous piles of paper documents by providing you with a database that can save all digitized files online. Moreover, it enables storing documents that are scattered all over different services (Dropbox, OneDrive, Google Drive, email attachments, etc.) of your employees in a centralized place.
Service for document distribution
The distribution process of documents via different channels is rather inefficient, out-of-date, and error-prone. Any highly-functional DMS can automate and simplify this process for you so that you may easily keep track of invoices, dunning letters, and other crucial papers sent within and outside the company.
General overview of DMS
Software for document tracking
Every DMS allows you to track the exact location of the required document easily by indexing documents. This DMS feature also includes tracking of version, and changes within the document.
Solution for workflow management
Highly-advanced DM systems enable flexible management of your business workflows. It means that you may save time and resources on numerous ineffective steps to execute a particular task. A health management system allows you and your employees to collaborate on a certain project, make adjustments, set notifications, etc., quite easily and without loss of time.
What’s in the box? The internal structure of a modern document management system
Having examined the essence of healthcare management software in general and the cases when it should be integrated, let’s take a closer look at its internal structure.
The capabilities of any full-fledged medical software system, such as document storage, capture, indexing, retrieval, workflow configuration, security, etc., depend on its architecture. Its components may vary; however, they don’t differ drastically. Thus, let’s take a look at the following core ones:
- Permission levels;
- Data storage, also called a central repository, with database and file server;
- Interface for users’ interaction with software;
- REST APIs to access centralized documents repository from different places and other clients.
The functional components above represent the standard architecture of the majority of health management systems and aren’t limited to them. In the picture below, you may see an example of classical DMS architecture:
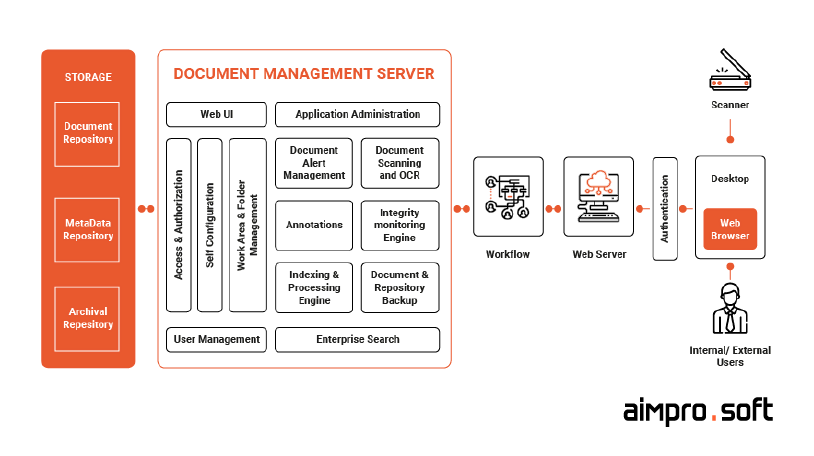
Example of standard DMS architecture
From theory to practice. Check out our step-by-step guide on how to build a medical DMS.
The DMS system’s internal structure and its functions show us that the integration of such software is not only desirable but is a must in many spheres. Banking, human resource management, legal field are just a few examples where such technology is highly applicable. Healthcare is probably the one that squeezes out others since it requires the highest level of security for its documentation. So let’s examine the role that healthcare DMS software can play in the medical industry.
Top red flags: how to identify when it’s time to integrate a robust DMS
As you may see, a medical management system is an integral software for those businesses where efficient work with documentation is the highest priority. However, what are the indicators that signify the moment that it’s about time to integrate such technology? As a healthcare software development company with more than 10 years of experience in the healthcare domain, we have encountered multiple cases of such indicators. Check out the list of the most critical scenarios when DMS implementation is highly recommended.
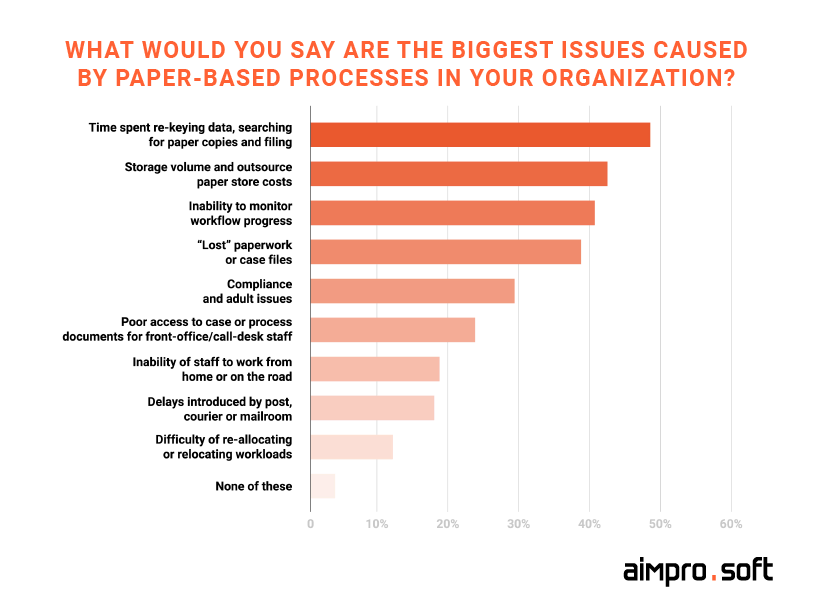
Major issues caused by paper documentation.
Your business is growing by leaps and bounds
If your business is rapidly transforming from a small startup into a mid-sized enterprise with more than 300+ employees, this is the first sign you’ll require a DMS soon. As the paper documentation flow is going to increase, so will the odds of human errors, paper loss, and insufficient document management. The integration of DMS medical software is a great preventive measure before such incidents may potentially happen.
Your current document management strategy is ineffective
If the productivity of your business is dragging because of the ineffectively working document management strategy, then it’s time to integrate a properly chosen DMS. It can automate daily paperwork processing routines, hence, decrease the time for process completion. Moreover, this technology allows employees to work with the same document simultaneously without waiting for other departments to make amendments.
Your company needs to reduce costs on document management
According to the survey, U.S. companies spend almost $8 billion every year to manage paper documentation, and only 18% of all businesses consider themselves paperless. If your expenditures on paper, file cabinets, shipping of paper documents, etc., are higher than you want them to be, healthcare management system integration will be a perfect solution in such cases. Moreover, it allows reducing costs in case you are renting physical paper storage.
Your employees spend too much time on document retrieval
Healthcare documentation management software may also be rather useful in case your employees waste lots of time retrieving necessary documents. This may happen due to various reasons: physical papers are misplaced, documents are lost, etc. Such risks can be diminished since such software labels files to speed up the search.
Your company faces regulatory compliance challenges
A properly designed document management strategy and software that enables its implementation will help you follow regulatory compliance without putting your business at stake.
Contact us, and we will examine your organization’s needs and recommend the right type of software to meet them.
CONTACT USImportance of a document management system in the healthcare industry
Representatives of the medical sphere face a great amount of paperwork: numerous medical history records, living wills, insurance cards, and test results. Therefore, fast document retrieval in case of emergency or enabling high-level security of the documents is the primary asset for any medical institution. Let’s take a closer look at those reasons that prove the importance of a document management system for the healthcare industry:
Benefits of medical document management software
Security first
The safety of your patients’ documents, health records, and internal documentation related to your organization, in general, may be vulnerable to all kinds of risks if the document management strategy is ineffective or absent at all. Natural disasters, human error, and technical issues (security breaches, data leakage, etc.) can significantly damage your papers. If such incidents occur, you can easily lose the trust of your patients, ruin your reputation and go out of business as a result. Due to this reason, the usage of medical document management software is highly important to eliminate such threats.
The management of the workflows
The fewer steps your personnel take to complete particular tasks related to all work processes and activities, the more seamless and efficient the workflow is. The importance of DMS technology in medical organizations is explained by an outstanding capability to manage workflows. It automates the whole process, allows different people to work on the document simultaneously, reduces the time for task completion, and directly influences the whole productivity of the organization.
Regulatory compliance
One of the most crucial reasons why healthcare organizations may require document management software for medical records is due to the necessity to adhere to regulatory compliance rules related to your field (HIPAA, HITECH, NHS, etc.). Revocation of licenses, fines, loss of customer trust, and sometimes even such severe sanctions as criminal consequences. The medical document management system ensures your compliance by providing a secure repository for data storage, docs permissions, version control, etc.
Learn more about the ways to make your healthcare records compliant with medical regulations.
We have examined the most crucial reasons that explain why the usage of the electronic document management system in healthcare is highly important. Now it’s time to see what are the actual benefits that such software can offer.
Top 5 benefits of a medical document management system
At this point, you have probably figured out what those gaps are in your healthcare organization management system but require more proof of how exactly healthcare document management software may benefit you. For this reason, the list of killer advantages is here for you:
Increased productivity
Fast access to documents, simplified form and paper filling, advanced full-text search, etc., are those factors that boost the productivity of your enterprise with a highly-functional medical electronic document management system. A properly chosen and adapted to your business needs document management system can boost the efficiency of all the internal processes and enhance the productivity of your personnel accordingly.
Accelerated access to documents and fast retrieval
Since a medical document management system offers its users the ability to label and index papers, it indicates the exact route where the document may be found. Moreover, since the documentation is located in centralized storage, their retrieval becomes an efficient “one-click” procedure.
Enhanced security
When you ask what is medical management software’s key benefit, it’s arguably the better security of your documentation that it can offer. DMS type of software can be easily adapted to your security strategy via:
- RBAC (role-based access control) — allows administrators to configure access to different files and build a structured hierarchy of permissions and restrictions to particular files;
- backups — DMS provides you with the ability to prevent data loss by performing automatic backups and keeping them in special storage folders separate from your organization’s database;
- DR (disaster recovery) — a properly chosen DMS allows you to configure a DR plan according to your needs, obtain access to the documents and health records after the disaster, prevent data loss, and minimize recovery time;
- data encryption — this DMS feature is a must for your security plan since it makes the information unreadable to those who don’t have the necessary credentials;
- audit trails — healthcare document management software enables you to track the activity of your employees related to the documents. Audit trails show your user’s ID, the exact time when the document was accessed, and the set of actions performed with the document. Such a DMS feature is rather valuable for the healthcare industry since it allows you to keep an eye on all changes across the system.
The features above are not limited to the ones mentioned. They depend on what DMS software you will choose to adapt to your business needs.
Flexibility
Even though you buy a DMS solution with OOTB (out-of-the-box) functionality, you still may add any required features and customize the platform according to your needs. It allows you to manage all your documents seamlessly and in less time than paper documents would require.
Reinforced consumer trust
If your healthcare organization works seamlessly, patients’ valuable papers are not constantly lost, the documents are retrieved at a rapid pace, and the customer churn will definitely bypass your institution.
Drop us a line, and our tech consultant will assess your requirements and design the integration strategy of such a system into your organization.
CONTACT USThe best document management software for healthcare in the market in 2023
Since numerous spheres so eagerly accepted DM systems, the newcomers have started entering the market rapidly while deep-rooted ones have expanded their functionality according to the market trends. As a result of such a DMS “boom,” healthcare organizations obtained one more pain while choosing the best-suited medical document management software.
Based on our expertise in the implementation of a document management system for the healthcare industry, we can guide you to the right choice:
- Measure the size of your healthcare organization, whether it is a small business or a mid-sized enterprise. Such a parameter will significantly affect the choice of DMS.
- Prepare thoroughly detailed business requirements that will help you form the vision of what problems the medical electronic document management system will solve.
- Figure out the required feature set that you want your DMS to have that will meet your business needs perfectly.
With all that in mind, proceed to the list of the best 5 medical DM systems that are highly popular on the market. Their description will help you understand how to choose a proper one and which type may be best for you.
| DMS | Best for | OCR | Workflow management | Unique features |
|---|---|---|---|---|
| Alfresco | any size of the medical enterprise (from 100 named users up to 1000) | advanced and easily configured | highly customizable | – a great number of collaboration tools – advanced security controls – a huge number of extensions – simplified creation of customer-specific UI – greatly designed extension architecture |
| PaperTracer | small healthcare businesses, mid-size businesses, enterprises | easily configured | customizable only in midsize and enterprise package | – e-signature capability – advanced reports and dashboards – redlining – the creation of custom templates |
| M-Files | small healthcare businesses and medium-sized ones | advanced OCR extensions | standard tools for workflows customization | – advanced search filters |
| FileHold | medium to large-sized healthcare organizations | easily configured | standard tools for workflows customization | – allows users to customize permission setting – Microsoft Office application integration |
| PinPoint | scalable for all business and enterprise sizes | easily configured | standard tools for workflows customization | – automatic workflow notifications for staff tasks |
Having examined our top medical electronic document management systems, let’s dive into the current market landscape and figure out what trends it has prepared for us.
Medical document management systems market trends
According to the statistical overview, the DMS market was estimated at $3.88 billion in 2019 and will reach $7.65 billion by 2025, at a CAGR of 12% over the outlook period 2020 — 2025. The statistics show that the integration of DMS software into enterprises is growing rapidly. This happens because numerous organizations are transforming their out-of-date strategies and opting for innovative software. Therefore, the market became segmented by three core parameters: mode of deployment (on-premises and cloud), end-user (entertainment, government, healthcare, insurance, legal, etc.), and geography.
Besides, the sector of medical records management software has also evoked the rise of trends that dictate how this software should be developed to meet the requirements of the end-users perfectly. Thus, follow the list below to see what are the top 5 tendencies in the healthcare document management system industry.
Make security great again
The digitization of paper documentation in healthcare eliminated a number of problems (paper loss, human error, etc.); however, it gave rise to different security risks, such as hacking, security breaches, and many others. Such threats created a tendency that encourages those who integrate such technology to choose wisely the one with top-of-the-line security features and those who develop electronic document management systems in healthcare to reinforce their security aspects via:
- full audit trail on all types of documentation within the enterprise;
- automated backups;
- role-based access control;
- compliance with the required healthcare regulations;
- data encryption;
- files archiving, etc.
Due to this recent trend, numerous medical companies started paying attention to cybersecurity and personnel training for the proper use of healthcare management systems.
Database security for medical document management system
Let’s not forget about mobile access
Modern medical professionals can be in a constant rush; hence they require constant access to the organization’s documentation. This aspect created the necessity to make DMS health technologies available on mobile devices. A user-friendly interface and robust design have become of high priority during the development of DM systems to make this software more profitable.
You might as well want to dive deeper into healthcare app development.
AI invasion
The performance even of the most highly-functional DMS healthcare software may seem not sufficient to keep up with the growing needs of healthcare organizations. To solve this problem, an increasing number of DM systems have been modified with the help of AI (artificial intelligence).
With the help of AI, document management systems obtained an OCR feature that enabled this software to read, process, and automatically classify documentation without human intervention. Moreover, an AI-powered electronic document management system in healthcare may provide faster data extraction and structuring. For instance, Google’s Document AI transforms unstructured data into structured documentation, automatically saving you and your personnel money, time, and effort.
It’s time to tune your collaborative work
Successful collaboration in a medical organization is of high importance. To provide healthcare enterprises with seamless and productive functioning, software companies have started upgrading their DM systems’ functionality. For example, Alfresco offers its full-fledged ECM with a great set of collaborative tools (smart folders, version tracking, discussion threads, etc.) that can significantly accelerate the internal processes of the whole medical organization.
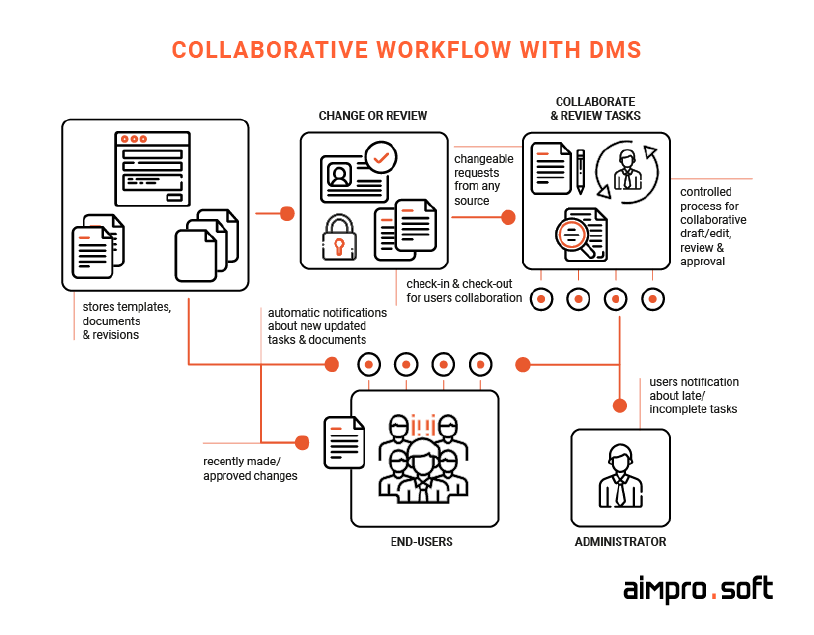
Example of collaborative work healthcare document management software
The smarter automation you have, the easier your life is
Similar to AI, RPA (robotic process automation) greatly speeds up and simplifies workflow-related processes and document management in general. The most prominent thing about RPA is that it can track and replicate human work with documents decreasing the time spent by personnel on various monotonous activities.
Our experience in healthcare document management system development and implementation
A real-life example can be the only way to understand the actual advantage of such software integration to the healthcare organization. Hence, continue reading this section, and you will learn more about the Aimprosoft approach to DMS development.
The customer turned to our services with the mere idea of creating a clinical trial DMS that would streamline all processes related to document capture, editing, tracking, sharing, and storing. Besides, the intention was to optimize workflow management among users and reduce error probabilities. Based on our previous expertise in healthcare DMS software implementation with Alfresco, we understood that the platform was the perfect choice to create a highly-functional electronic document management system.
Our fruitful cooperation with the customer resulted in 3 successive releases of the product. Each subsequent version of the customer’s document management software for medical records was developed to modify and enhance the functionality of its predecessor. Thus, let’s take a look at the evolution of our DM system:
Chapter 1: The rise
Firstly, we transformed quasi-paper inefficient document management processes, inefficiently managed DCRs (Document Change Requests), and weakly controlled document post-approval changes with the help of Alfresco OOTB functionality. Among the key features that we implemented in the first version, there were: a standard toolset for workflow management, document versioning, and easily manageable audit trails. We also enabled seamless processes of document approval and rejection.
The security, in turn, was enabled via in-place features that prevented unwanted access. As a result, we developed a document management system with basic functionality and workflow.
Chapter 2: The enhancements
The second version was marked by significant improvements in controlled documentation management, an audit trail of document activity, and the security of the overall system. Since Alfresco OOTB functionality wasn’t enough to make the whole solution fully functional, we modified the UI by creating collapsible panels and an easily operated navigation bar with menu icons. We also enabled users to define whether the amendments made within the document were major or minor, and attach comments to these changes.
Moreover, users could seamlessly access the document’s full version history and track the required one. The security of the system was also modified since the previous version of the DM system didn’t fully enable high-level safety of the documentation workflow within the enterprise. Hence, for authorized users, we extended the standard way of setting security permissions on documents and folders. It was also possible to classify the document security level by using a ‘Classification’ field that offered users 3 standard options: confidential, sensitive, and public.
Chapter 3: The ideal solution
The necessity for the final improvements occurred due to the growth of our client’s enterprise. We extended the standard tools set for workflow management by enabling users to set deadlines for workflows and configure diverse characteristics of the workflows they work with. Role-based access control became more complex and differentiated. For instance, only Workflow Managers could start and filter the ongoing assigned workflows, and approve the final version of particular documents.
The security aspect of the client’s healthcare document management system was also enhanced significantly; namely, we reinforced the security of audit trails by restricting the ability to modify, disable, and delete them. Also, in order to eliminate the risks of accident document deletion, we implemented a special request field where all users could provide the reason for the document/folder deletion.
Contact us, and we’ll evaluate your request and find the right approach for your unique case.
CONTACT USOverall, after the third version, our customer obtained a highly-functional clinical trial DMS. Having gone through three iterations, the solution acquired those features that efficiently enable document safety and seamless workflow management.
Conclusion
The necessity to automate your healthcare organization is now higher than ever before. Likewise, the opportunities to integrate efficient technologies that will take the pain related to the document management processes away are greater than ever before. Thus, if you are ready to streamline your business with the right medical document management software but don’t know how drop us a line, and our specialist will certainly guide you in the right direction.
FAQ
What regulations should a medical DMS be compliant with?
Regulatory compliance is vital for such software. Firstly, the system should maintain complete HIPAA (Health Insurance Portability and Accountability Act) compliance which means your DMS addresses all technical, physical, and administrative safeguards outlined by HIPAA Security Rule. Secondly, The DMS of the US healthcare organizations should follow HITECH (Health Information Technology for Economic and Clinical Health Act) software compliance principles that address the privacy and security concerns related to the electronic transmission of health information.
It’s also required to comply with the NHS (UK National Health Service) in case the software is developed for the British market. Finally, don’t forget about GDPR (General Data Protection Regulation) guidelines that require healthcare software to provide high security for all gathered and processed personal data.
Should medical DMS be integrated with other software within the organization’s infrastructure? Or do they function separately?
Document management systems are frequently used as standalone solutions. However, organizations may also want to enhance DMS capabilities by integrating it with other software. To understand whether the integration of your medical DMS with other existing services is worth it or not, it’s necessary to define what gaps the leveraged solutions cover and what value you will obtain from this integration. For example, let’s examine the integration of medical DMS and a healthcare CRM system.
The CRM contains all necessary patient and business-related information, and its main goal is to help businesses enhance patients’ loyalty to a particular healthcare organization and reduce churn. If we take DMS capabilities and empower them with CRM, we will create a fully integrated system that can help healthcare organizations significantly reduce costs spent on managing multiple disparate services, streamline documentation workflow, obtain more accurate insights, etc. This is an example of DMS integration with the other solution that can be rather fruitful for healthcare organizations.
How long does it take to develop a medical DMS?
There are two possible scenarios of medical DMS development that influence the time that will be spent on such software creation. The first option is the customization of the already existing solution on the market. Its essence is to take the OOTB functionality of the chosen software and adjust it to the needs of the healthcare organization. The estimation of time that will be spent on software customization is based on the size of the medical organization, business and technical requirements, and the extent to which the software needs to be customized.
Another scenario is to build such software from scratch. This option is more time, effort, and cost-consuming and takes at least 1 year to develop it. For small-sized medical organizations, this option may be quite challenging in terms of budget; hence, it is more suitable for mid and large-sized organizations.
What is the best time to start using such software?
There are numerous factors that influence the necessity of medical document management software integration into the company’s infrastructure, such as the size of the company’s workforce, the volume of the documentation flow, etc. To avoid inefficient document-related processes, slow document retrieval, high costs on document storages, and other challenges that may emerge without such software, we advise starting using documentation systems in healthcare facilities from the very beginning of the institutions’ existence since the volume of documentation is increasing rather rapidly in the healthcare industry.
How much do you charge for building a custom healthcare DMS?
The desired functionality and complexity of its implementation, degree of customization, deadline, and budget are all significant factors that can impact both the time and cost of DMS software development. For example, if you choose custom development from the ground up, it could take several years to execute this solution. On the other hand, if you choose a ready-made solution with a fixed pricing model, you may encounter unexpected installation costs. However, there is a customizable solution that we see as the golden mean between these choices in terms of both time and cost.
What is the importance of documentation in healthcare?
Documentation is essential in healthcare to ensure that patients receive safe, high-quality care and that healthcare providers are able to communicate and coordinate effectively to achieve optimal patient outcomes.